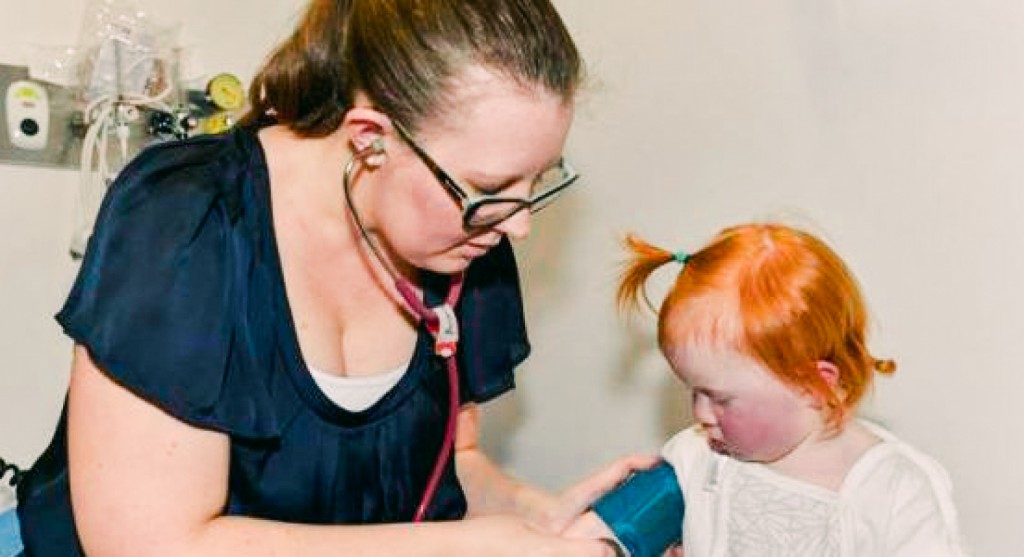
Project Overview
The Clinical Trials project is at the core of the Paediatric Precision Medicine Program’s mission to translate groundbreaking therapies to children with rare diseases and cancer.
This initiative facilitated the execution of clinical trials involving novel gene and cell therapies for pediatric patients with rare genetic diseases and cancer. By prioritising children in trial design and development, the project significantly expanded the number of pediatric clinical trials in NSW.
Furthermore, it bolstered the capability of clinicians and researchers to identify potential trial opportunities, deepened their understanding of trial methodology and protocol design, and formulated an education, implementation, and service model to seamlessly deliver early-phase and innovative trials within the NSW healthcare system.
Collaborations
- Children’s Cancer Institute (Australia)
- Children’s Medical Research Institute (Australia)
- University of Sydney (Australia)
- University of New South Wales (Australia)
- Pharmaceutical/Biotechnology
- Sydney Children’s Hospitals Network, Clinical Research Centre (Australia)



 Back to Research
Back to Research