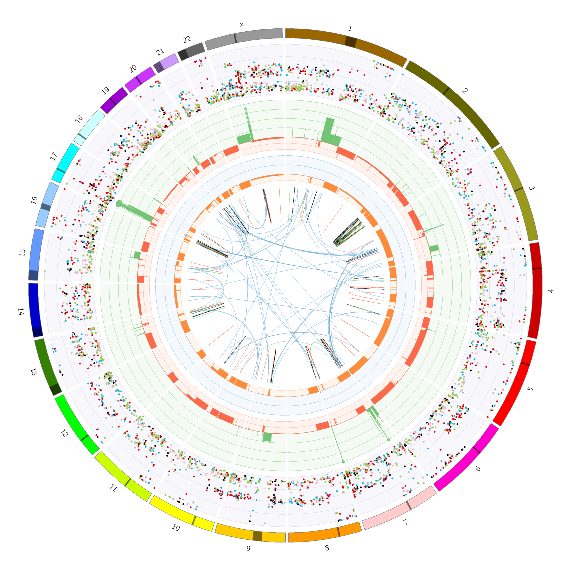
Our Computational Biology team joins CAVATICA to collaborate on the exchange and analysis of paediatric cancer data .
CAVATICA is an international cloud-based platform that allows partners to collaborate through the sharing and analysis of paediatric cancer data.
In 2018, the Australian Bioinformatics Data Commons (AusBioCommons) project, funded by the Australian Research Data Commons (ARDC) was launched, to establish an Australian arm of the CAVATICA platform.
The Precision Medicine Program Team Computational Biology Program (PPM2) has been working closely with AusBioCommons by leading a flagship research project that will integrate RNAseq data from Australian patients with that from 1000 patients from the United States.
As part of the AusBioCommons collaboration, the computational biology team team hosted as series of workshops in early February at the Children’s Cancer Institute, to provide training on the use of the Australian CAVATICA platform and the development of genomic analysis applications. The workshop included Dr Allison Heath, the Director of Data Technology and Innovation and two engineers from the US based CAVATICA platform. A webinar was delivered from the workshop to showcase the potential of the platform to Australian users.
This exciting partnership will see the establishment of the Australian Bioinformatics Commons Paediatric Pathfinder Project enabling data sharing between Australian and American researchers. The first project will see RNA-Seq brain-tumour data from the ZERO cancer program (Aus) merge with the Children’s Brian Tumour Tissue Consortium (USA) to identify novel brain tumour subtypes.
The early outcomes of this project have been substantially reduced analysis costs, international knowledge sharing and collaboration. Importantly, AusBioCommons may become the preferred way to enable sharing of large-data precision medicine data generated by projects across the Luminesce Alliance, including the Cancer Predisposition Screening Program (PPM3).