New research aimed at developing ground-breaking treatments for children with rare diseases, cancers and neurodevelopmental disorders have been funded by Luminesce Alliance.
Investment of more than $300,000 in the three 12-month innovation projects as part of our precision medicine Enabling Platforms strategy, has the potential to revolutionise the understanding and treatment of conditions including blinding eye disease, Rett syndrome and hard to treat cancers.
“These innovative, collaborative research projects bring together experts working towards our mission of transforming the prevention and treatment of childhood illnesses,” said Luminesce Alliance Executive Director Anastasia Ioannou. “Collaboration is the cornerstone of Luminesce Alliance – our partners pool their collective expertise to provide the critical mass needed to work towards transforming paediatric research.”
Genetic drivers of Rett Syndrome
Associate Professor Wendy Gold, Head of the Molecular Neurobiology Research Laboratory at Kids Research will lead a study aimed at unlocking the mystery of the rare severe neurodevelopmental disorder Rett syndrome. Her team will use integrative approaches to investigate the disease drivers of the disorder, as well as improving methods of predicting its severity and responses to treatments.
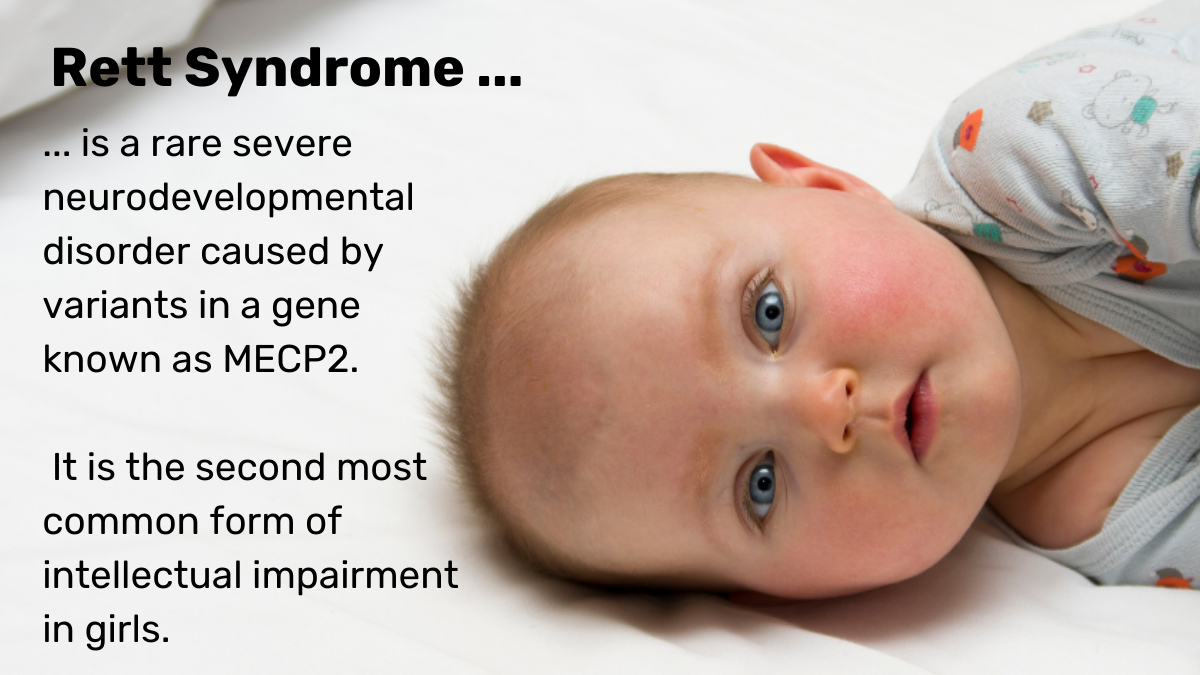
Rett syndrome is a rare severe neurodevelopmental disorder, caused by variants in a gene known as MECP2. It causes babies to lose movement and communication and affects one in 10,000 children, mainly girls, including 430 in Australia. The syndrome is hard to diagnose and is often confused with autism or mitochondrial disease as girls with Rett syndrome have symptoms that overlap with characteristics of these disorders.
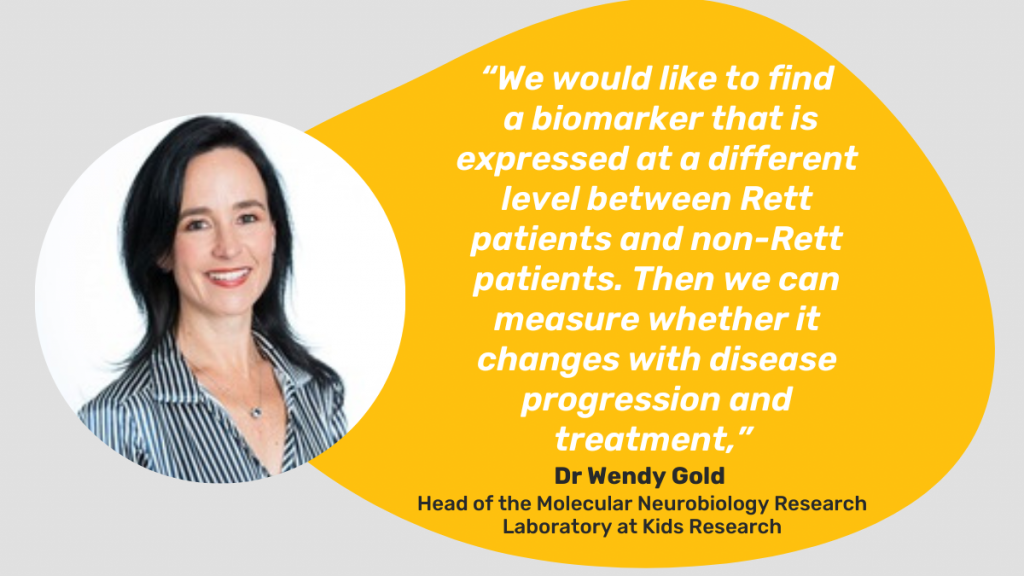
Researchers around the world are desperately looking for a treatment for Rett syndrome, but despite many clinical trials, there is no cure. One of the reasons clinical trials have failed is that no clinically useful biomarkers have been identified for Rett syndrome.
The new study – Integrative omics: A novel approach to unravelling the complex panoramic landscape – aims to identify disease drivers, drug targets and clinical biomarkers that can predict disease state, disease severity, and treatment efficacy.
A/Prof Gold said the ultimate aim is for clinicians to be able to test for these biomarkers to aid in the diagnosis, and for scientists and pharmaceutical companies to have a reliable measure of disease improvement in clinical trials.
Read more about A/Professor Gold’s Luminesce funded research
Other investigators collaborating on this project include Dr Mark Graham, CMRI, Adviye Ayper Tolun, SCHN, Ashley Hertzog, SCHN, A/Prof Carolyn Ellaway, SCHN, Alexander Wykes, CMRI, Florencia Haase, SCHN, Dr Brain Gloss, WIMR.
New drugs to treat childhood cancers
A study looking speeding up and streamlining the discovery of new drugs to treat childhood cancers will be led by Associate Professor Paul Ekert who is group leader of Computational Biology at the Children’s Cancer Institute (CCI).
Outcomes for children with the most difficult-to-treat cancers remains dismal due to the lack of effective standard treatment options. Combining big data, computational strategies and novel experimental approaches in the laboratory, the project aims to identify molecular drivers of childhoods cancers, potentially leading to new treatments targeting specific genes.
This project will address a critical gap in this process by using computational biology methodologies to sift the vast amount of genetic information being generated about childhood cancers.
“Over the last four years, CCI has collected and profiled the genetic make-up of over 500 high-risk paediatric tumours through the Zero Childhood Cancer Program,” A/Prof Ekert said.
“This provides us with an unprecedented dataset, from which we can gain insight into the specific molecular features and potential drivers of some of the most intractable paediatric cancers.”

Other investigators collaborating on this project include Dr Antoine de Weck, CCI, Dr Ian Street, CCI, A/Prof Greg Arndt, CCI, Dr Antoinette Anazodo, SCHN, Dr Rebecca Poulos, CMRI, Dr Tim Failes, CCI.
Read more about the Luminesce Alliance Computational Biology research
Restoring vision using stem cell transplantation
Restoring the vision of patients with blinding eye disease using stem cell therapy is the aim of the third study, led by Dr Anai Gonzalez Cordero, Group Leader Stem Cell Medicine at the Children’s Medical Research Institute (CMRI).
Millions of people worldwide live with severe degenerative diseases of the eye leading to progressive vision impairment and eventually total blindness. The majority of these inherited and acquired degenerative diseases affect the light-sensing tissue at the back of the eye, the retina, that contains the rod and cone photoreceptors and are untreatable.

The study will further Dr Gonzalez Cordero’s research into the possibility of restoring vision using stem cell transplantation.
“We have demonstrated proof-of-concept for the transplantation of mouse and human stem cell-derived photoreceptors to rescue visual perception in mice,” she said. “In this project, we will move this therapy closer to implementation.”
Read more about Dr Gonzalez Cordero’s research
Other investigators collaborating on this project include Prof Patrick Tam, CMRI, Dr Ngaire Elwood, Murdoch Children’s Institute.
Continue reading “Funding innovative collaborations to transform paediatric precision medicine”