Media Release: ‘Zero Childhood Cancer’ Clinical Trial Delivers Promising Results Within Its First 11 Months
The Zero Childhood Cancer program has today released initial results of its national clinical trial, revealing promising outcomes within its first 11 months.
Of the 129 children enrolled in the trial from across Australia with high-risk and relapsed cancers, 67% were provided with personalised treatment plans aimed at killing their unique cancer cells. For most children enrolled in the trial, there were otherwise few to no treatment options available to them.
Led by Children’s Cancer Institute and the Kids’ Cancer Centre at Sydney Children’s Hospital, Randwick, Zero Childhood Cancer is one of the world’s most comprehensive child cancer personalised medicine studies. The trial uses sophisticated genetic tests to scientifically analyse each child’s individual cancer cells to identify and recommend new personalised treatment options.
Associate Professor Tracey O’Brien, Director, Kid’s Cancer Centre at Sydney Children’s Hospital, Randwick says the trial is giving a small group of children a better chance of survival, where current treatment affords little hope.
“Zero Childhood Cancer is about using the best science we have to give hope to children with high risk cancer. We must try a different approach. Accepting the status quo means that 70% of these children won’t survive to celebrate another birthday,”
Associate Professor O’Brien said.
“Our early results are encouraging and as we learn more, I see future potential for targeted drug therapies to be used more broadly in all child cancers as a smarter way to achieve cure, while minimising therapy side effects.”
Executive Director of Children’s Cancer Institute, Professor Michelle Haber AM, said the Zero Childhood Cancer program is bringing us one step close to personalised medicine for all childhood cancers.
“Zero Childhood Cancer is giving unprecedented genetic and biological information for children with the most aggressive cancers. It is arguably the most comprehensive personalised medicine program for children with cancer.
“The information we gather will not only benefit children on the national clinical trial but will inform new discoveries and further clinical trials that we believe will impact all children with cancer in the future.”
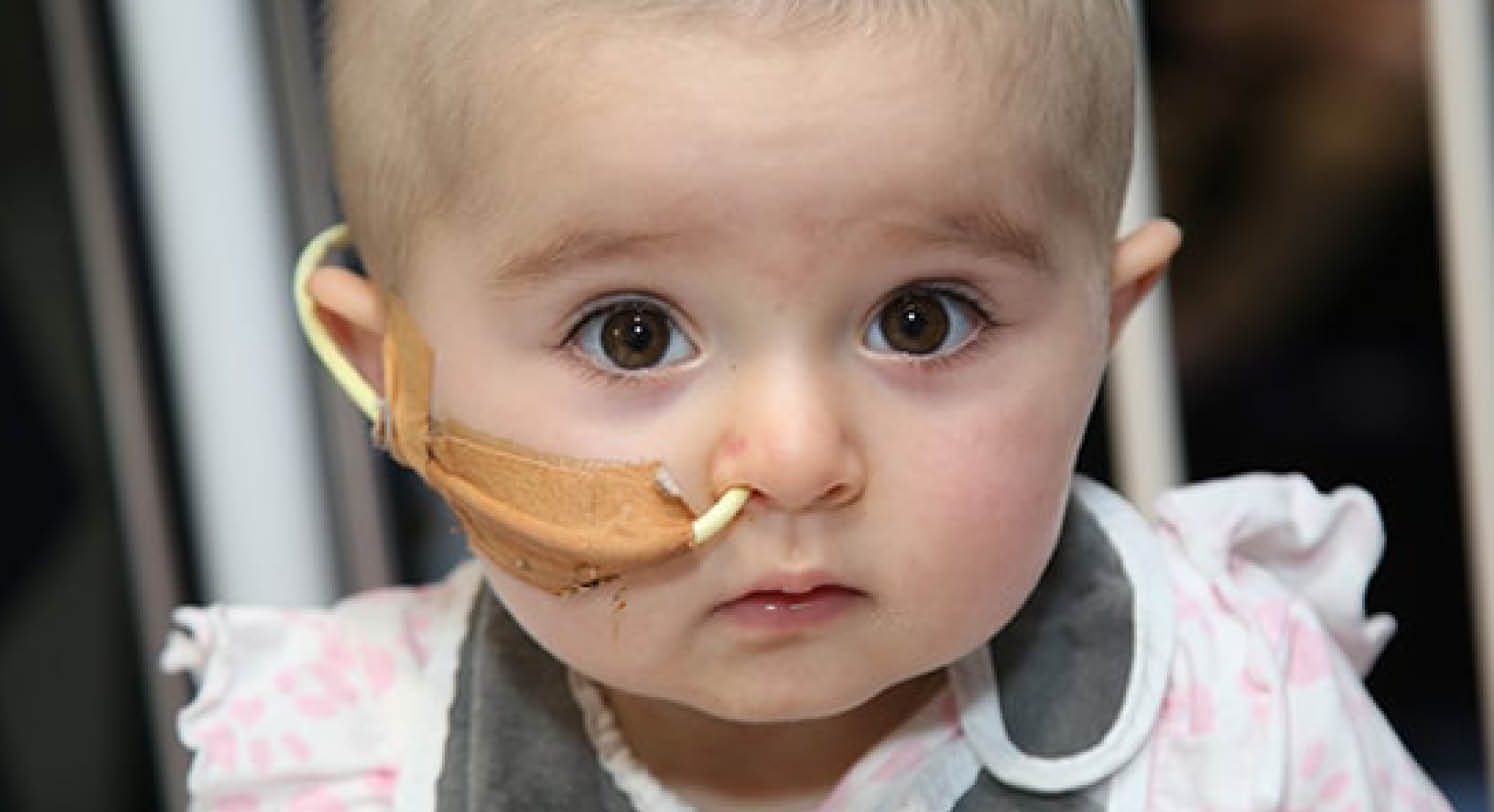
No story exemplifies the impact of Zero Childhood Cancer more than that of Ellie. At just 11 months old, Ellie was diagnosed with infantile fibrosarcoma, a rare and aggressive tumour that was resistant to chemotherapy. The tumour was so large that she was on life support.
Following sequencing of the entire genetic material of Ellie’s tumour through our partnership with the Lions Kids Cancer Genome Project, the whole genome sequencing identified the specific genetic change likely to be driving Ellie’s cancer. The Zero Childhood Cancer team were then able to identify a new drug that specifically targeted that particular genetic change. The drug was sourced, after four weeks of treatment, Ellie’s cancer had shrunk enough for her to be taken off life support and breathe independently. Six weeks later, Ellie was home.
Ellie’s parents, Mina and Rob, know their daughter is only here today because of the Zero Childhood Cancer program.
“We were told to think about saying goodbye, she was so sick we didn’t even know if she would reach her first birthday. Now, to be celebrating her second birthday, when she is such an active, boisterous and energetic two-year-old is beyond our wildest dreams. We can’t thank the teams at the hospitals and research centres involved in the Zero Childhood Cancer program enough,” Mina said.
Outcomes of the Zero Childhood Cancer program over the past 11 months:
- 128 children registered for the trial after just 11 months, each of these are children with an aggressive cancer that is identified as having less than a 30% chance of survival
- Of these, 36% have been enrolled at the time of relapse, 38% at diagnosis and 26% with progression of disease
- In terms of cancer types, 36% have brain cancer, 29% sarcoma, 13% leukaemia, 6% neuroblastoma and 16% other rare cancers
- For 67% of children a personalised treatment plan has been recommended
- Average turnaround time from receipt of samples to personalised treatment recommendation is 9 weeks
Despite the dramatic increase in childhood cancer survival rates over the last sixty years from virtually 0 to 80%, three children and adolescents still die every week in Australia from cancer.
For further information about Zero Childhood Cancer visit www.zerochildhoodcancer.org.au
For more information on Trial outcomes, Ellie’s story and supporters of the Zero Childhood Cancer program please click here.